CAR-T’s clinical and manufacturing considerations highlight the importance of earlier treatment in relapsed or refractory multiple myeloma (RRMM).
Fewer patients MAKE IT TO THE NEXT LINE OF THERAPY 1
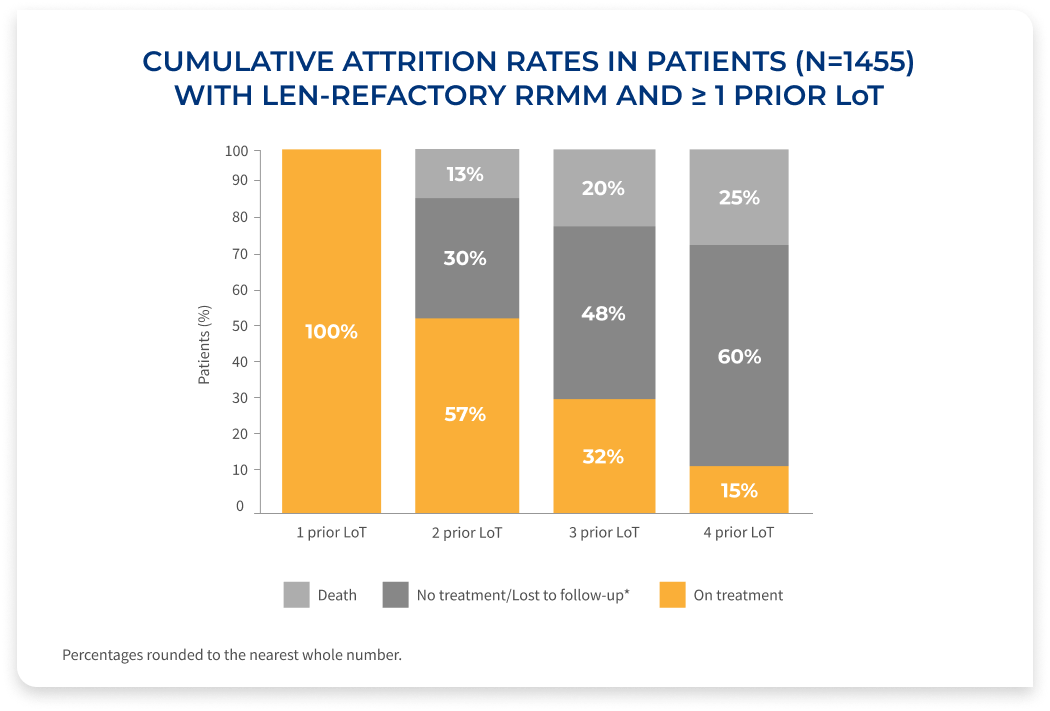
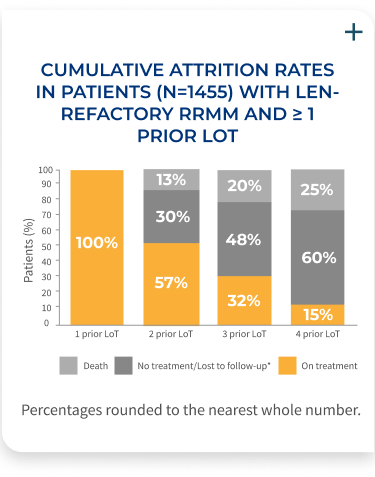
The percentage of patients on treatment decreases with each LoT, demonstrating the need for efficacious therapies at first relapse in RRMM
Results of a retrospective database analysis (January 2016-April 2022) of 1,455 RRMM patients with 1-3 prior LoT, ECOG PS <2, who were exposed to PI and an immunomodulatory agent, and were considered len-refractory.
Study Overview
- Patients had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of <2 and were derived from the Flatiron Health de-identified US electronic health records database (January 2016–April 2022)
- Patients who are len-refractory were defined as having a change in treatment within 60 days of last lenalidomide therapy without lenalidomide as a component of the immediate next LoT. Index date was defined as the start of first therapy (index therapy) after a patient met the inclusion criteria
- Daratumumab/pomalidomide/
dexamethasone (DPd) was the most commonly used index regimen (13.2%), followed by daratumumab monotherapy (8.3%), Pd (7.7%), carfilzomib/
pomalidomide/dexamethasone (KPd) (7.1%), and Kd (7.0%)
CAR-T=chimeric antigen receptor-T cell; len=lenalidomide; LoT=line(s) of therapy; PI=proteasome inhibitor.
*Patients who received an active antimyeloma treatment and had ≥1 follow-up assessment but were subsequently lost to follow-up.
T-CELL FITNESS AND EFFECTIVE BRIDGING THERAPY CAN ENHANCE CAR-T OUTCOMES

T-cell fitness* is greater earlier in the disease course and may be associated with improved outcomes1-4
- T-cell exhaustion has been attributed to prior treatment exposures, such as alkylating therapies (eg, bendamustine) and underlying MM1-4
- CAR-T cells derived from T-cells earlier in the disease course may be associated with improved outcomes (manufacturing, cell expansion, and cytotoxicity)2,4

Effective† bridging therapy (BT) may help reduce AEs and
improve PFS of CAR-T therapy1,5
- BT is a recommended strategy to control disease and reduce tumor burden during CAR-T cell manufacturing, particularly in patients with aggressive or high-burden disease5,6
- BT may help minimize the risk of neurologic AEs after BCMA CAR-T therapy1,5
- Response to bridging has been associated with improved progression-free survival5
- Agents or regimens that the patient has not been previously exposed to are preferred for BT when possible1
- Patients in earlier-line settings may have a greater number of effective treatment options, allowing for optimal selection of bridging therapy1,5
Early referral for CAR-T cell therapy facilitates the collection of fitter T-cells and expands the options for effective bridging therapy1,5
AE=adverse event; BCMA=B-cell maturation antigen; CAR-T=chimeric antigen receptor-T cell; MM=multiple myeloma; PFS=progression-free survival.
*Based on prior treatment exposure, disease progression, and markers typically associated with T-cell fitness determined by flow cytometry.1,2,4
†Effective defined as reduction in tumor burden (typically ≥25% reduction).