Powerful Results
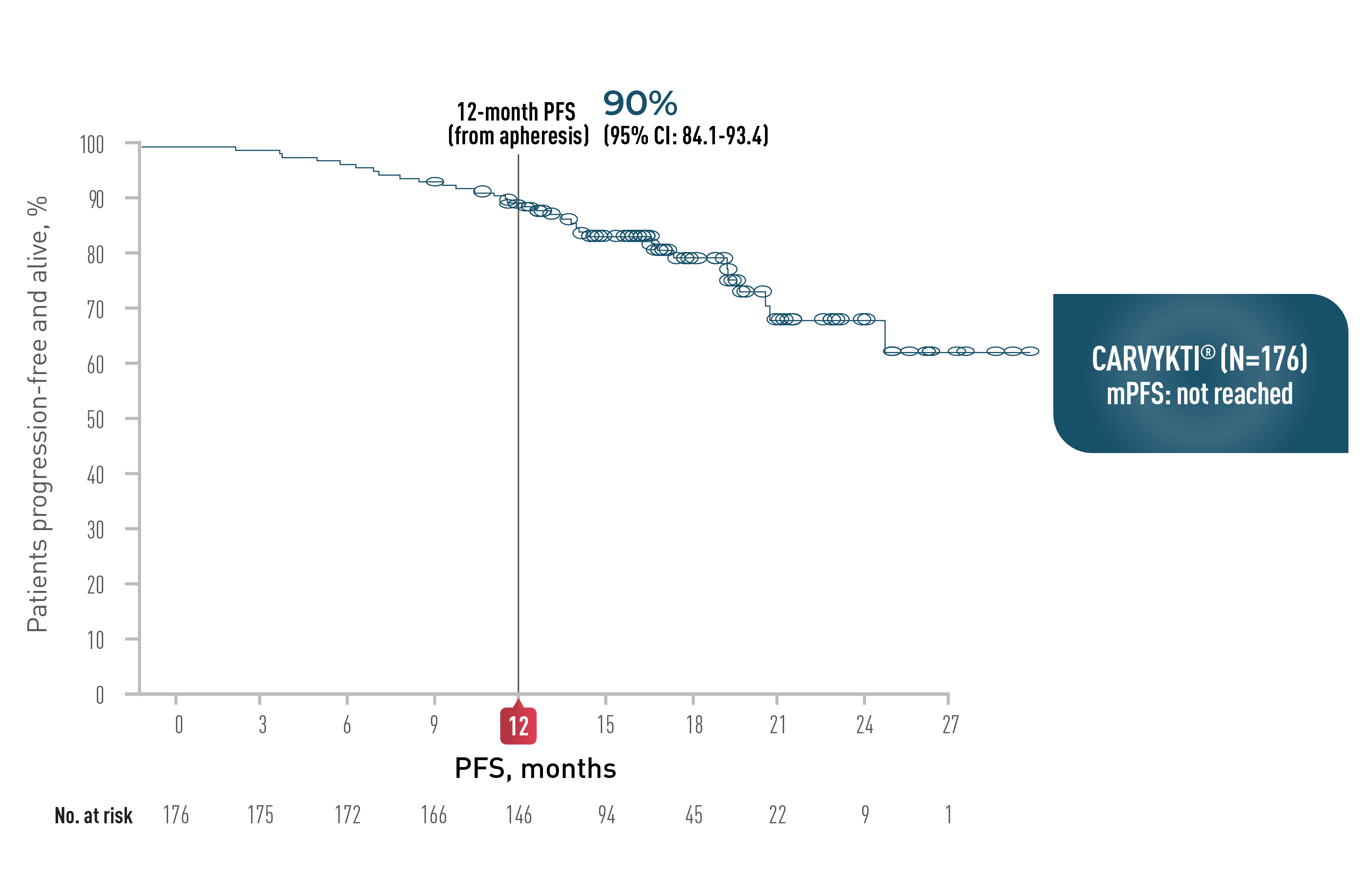
In CARTITUDE-4 at 15.9 Months
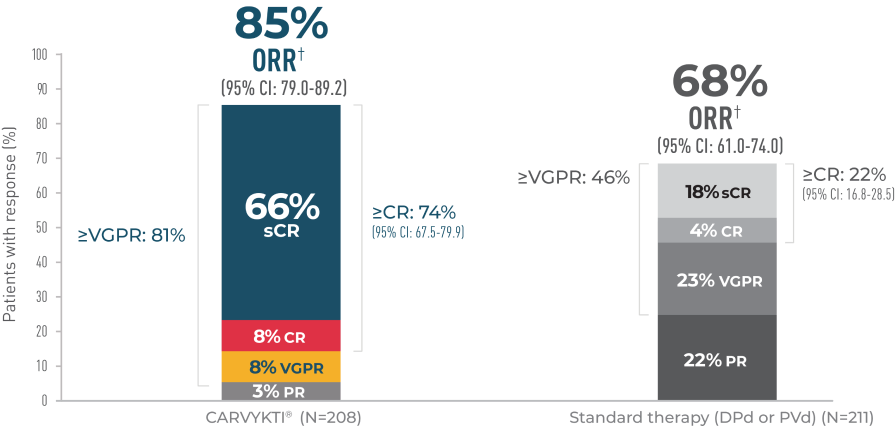
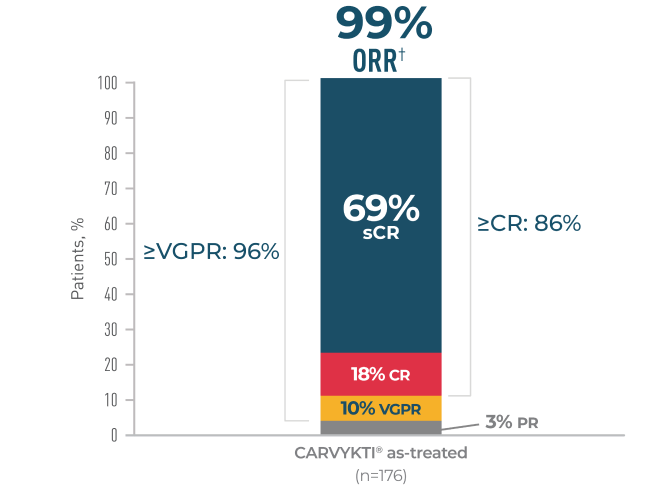
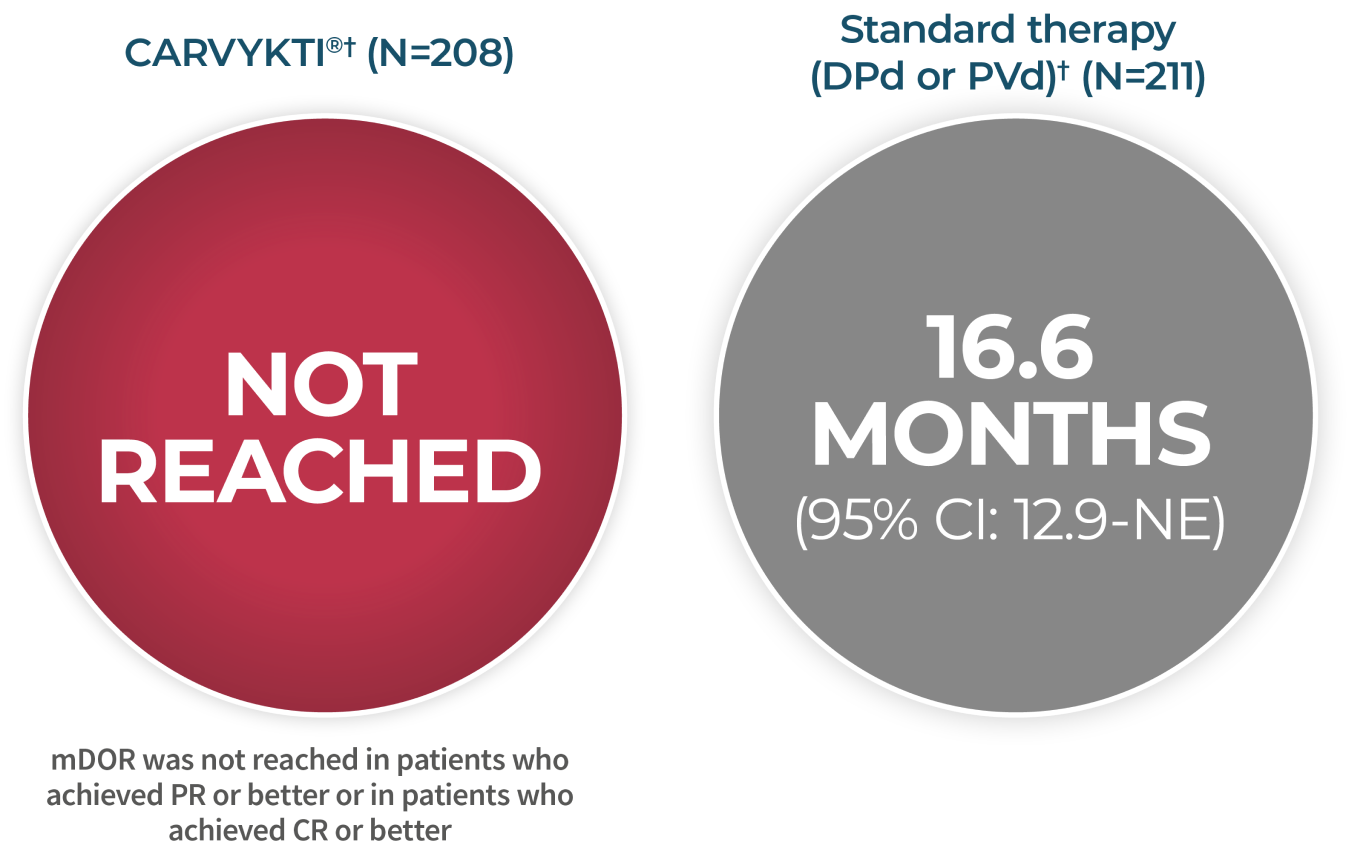
CARVYKTI® SIGNIFICANTLY PROLONGED Progression-Free Survival (PRIMARY ENDPOINT) vs STANDARD THERAPY (DPd or PVd)1*
PROGRESSION-FREE SURVIVAL
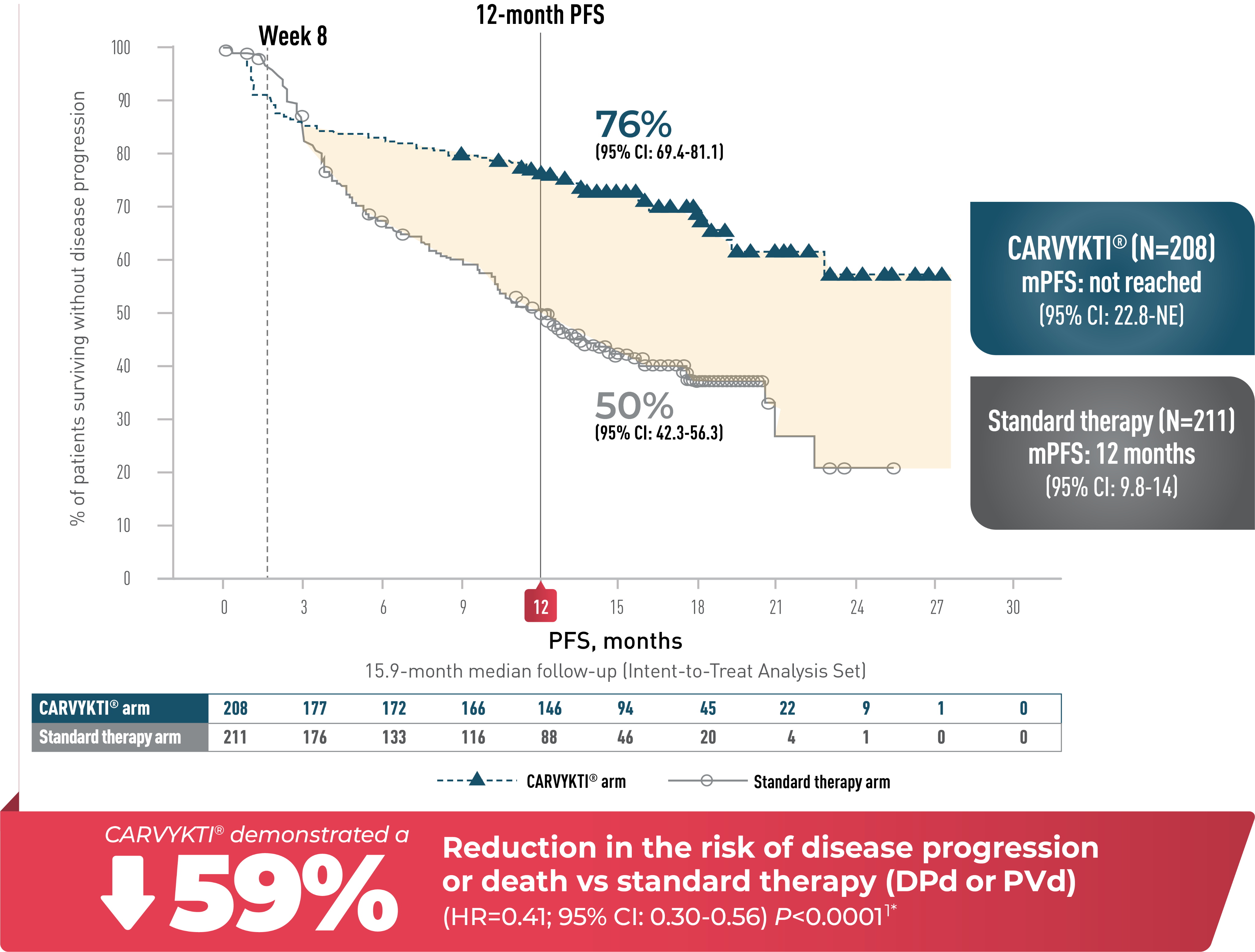
PFS SUBGROUP ANALYSIS
PFS SUBGROUP ANALYSIS
Percentages rounded to nearest whole number.
CI=confidence interval; DPd=daratumumab, pomalidomide, and dexamethasone; HR=hazard ratio; mPFS=median progression-free survival; PVd=pomalidomide, bortezomib, and dexamethasone.
*Median follow-up was 15.9 months in the Intent-to-Treat Analysis Set.