The safety data described in this section reflect exposure to CARVYKTI® in 285 patients with relapsed or refractory multiple myeloma: one randomized, open-label study with 188 patients in CARTITUDE-4 and one single-arm, open-label study with 97 patients in CARTITUDE-1. Because clinical trials are conducted under widely varying conditions, adverse reactions observed in clinical trials may not reflect the rates observed in practice.
BOXED WARNING
WARNING: CYTOKINE RELEASE SYNDROME, NEUROLOGIC TOXICITIES, HLH/MAS, PROLONGED and RECURRENT CYTOPENIA, and SECONDARY HEMATOLOGICAL MALIGNANCIES
- Cytokine Release Syndrome (CRS), including fatal or life-threatening reactions, occurred in patients following treatment with CARVYKTI®. Do not administer CARVYKTI® to patients with active infection or inflammatory disorders. Treat severe or life-threatening CRS with tocilizumab or tocilizumab and corticosteroids.
- Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS), which may be fatal or life-threatening, occurred following treatment with CARVYKTI®, including before CRS onset, concurrently with CRS, after CRS resolution, or in the absence of CRS. Monitor for neurologic events after treatment with CARVYKTI®. Provide supportive care and/or corticosteroids as needed.
- Parkinsonism and Guillain-Barré syndrome (GBS) and their associated complications resulting in fatal or life-threatening reactions have occurred following treatment with CARVYKTI®.
- Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome (HLH/MAS), including fatal and life-threatening reactions, occurred in patients following treatment with CARVYKTI®. HLH/MAS can occur with CRS or neurologic toxicities.
- Prolonged and/or recurrent cytopenias with bleeding and infection and requirement for stem cell transplantation for hematopoietic recovery occurred following treatment with CARVYKTI®.
- Immune Effector Cell-associated Enterocolitis (IEC-EC), including fatal or life-threatening reactions, occurred following treatment with CARVYKTI®.
- Secondary hematological malignancies, including myelodysplastic syndrome and acute myeloid leukemia, have occurred in patients following treatment with CARVYKTI®. T-cell malignancies have occurred following treatment of hematologic malignancies with BCMA- and CD19-directed genetically modified autologous T-cell immunotherapies, including CARVYKTI®.
CYTOKINE RELEASE SYNDROME (CRS)
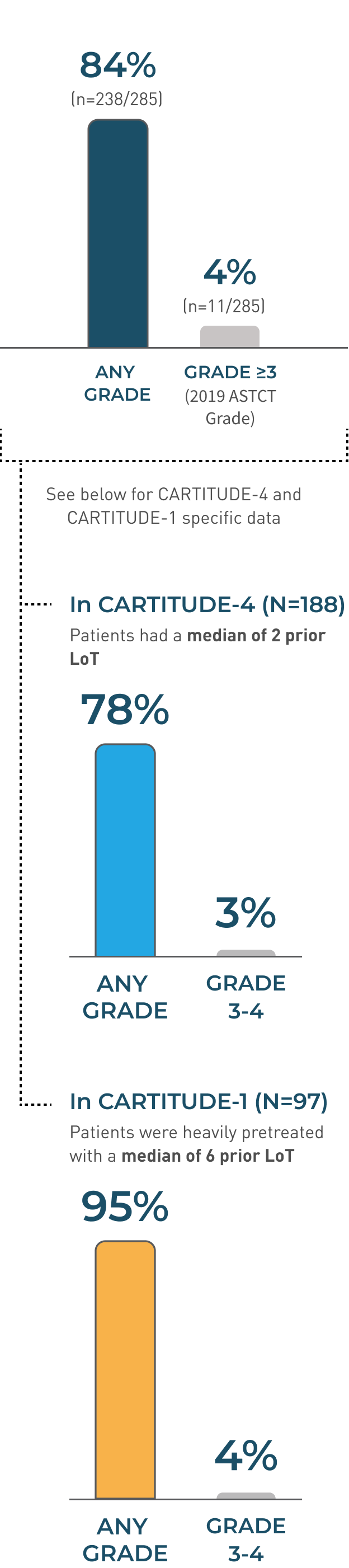
In CARTITUDE-4 and CARTITUDE-1 (N=285)1
CRS, including fatal or life-threatening reactions, occurred after CARVYKTI® infusion.
CRS Incidence Rates

CARTITUDE-4 and CARTITUDE-1
POOLED DATA (N=285)
Additional CRS Pooled Data
Median time to onset
7 days
(range: 1-23 days)
Median Duration
4 days
(range: 1-97 days)

CRS RESOLVED IN 82%
(n=195/238)
Monitor patients for
signs and symptoms of crs*
At least daily for
7 DAYS
after infusion
At least daily for
2 WEEKS
after infusion
Majority of CRS events were low grade (Grade 1/2 80%; 227/285), with 4% of patients experiencing Grade ≥3 (11/285)
- The median time to onset of CRS, any grade, was 7 days (range: 1-23 days)
- CRS resolved in 82% of patients with a median duration of 4 days (range: 1-97 days)
- The most common manifestations of CRS in all patients combined (≥10%) included fever (84%), hypotension (29%), and aspartate aminotransferase increased (11%)
- Serious events that may be associated with CRS include pyrexia, hemophagocytic lymphohistiocytosis, respiratory failure, disseminated intravascular coagulation, capillary leak syndrome, and supraventricular and ventricular tachycardia
- Identify CRS based on clinical presentation*
- Evaluate for and treat other causes of fever, hypoxia, and hypotension. CRS has been reported to be associated with findings of HLH/MAS, and the physiology of the syndromes may overlap. HLH/MAS is a potentially life-threatening condition. In patients with progressive symptoms of CRS or refractory CRS despite treatment, evaluate for evidence of HLH/MAS
- Of the 285 patients who received CARVYKTI® in clinical trials, 53% (150/285) received tocilizumab; 35% (100/285) received a single dose, while 18% (50/285) received more than 1 dose of tocilizumab. Overall, 14% (39/285) of patients received at least one dose of corticosteroids for treatment of CRS
- At the first sign of CRS, immediately institute treatment with supportive care, tocilizumab, or tocilizumab and corticosteroids
NEUROLOGIC TOXICITIES
In CARTITUDE-4 and CARTITUDE-1 (N=285)1
Neurologic toxicities, which may be severe, life-threatening, or fatal, occurred after CARVYKTI® infusion
Neurologic Toxicities Incidence Rates
CARTITUDE-4 and CARTITUDE-1
POOLED DATA (N=285)


Additional Neurologic Toxicities
MEDIAN TIME TO ONSET
10 DAYS(range: 1-101 days)
MEDIAN TIME TO RESOLUTION
23 DAYS(range: 1-544 days)
Neurologic TOXICITIES RESOLVED in 72%
(n=50/69)


Neurologic TOXICITIES RESOLVED in 72%
(n=50/69)
Neurotoxicity events resolved in 72% of patients with a median time to resolution of 23 days (range:1 to 544)
- 17% of patients had low-grade (Grade 1/2) events compared to 7% experiencing Grade ≥3
- 91% (63/69) of neurotoxicity events developed within 30 days
- Of patients developing neurotoxicity, 96% (66/69) also developed CRS. Subtypes of neurologic toxicities included ICANS in 13%, peripheral neuropathy in 7%, cranial nerve palsy in 7%, parkinsonism in 3%, and immune-mediated myelitis in 0.4% of the patients
Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS)
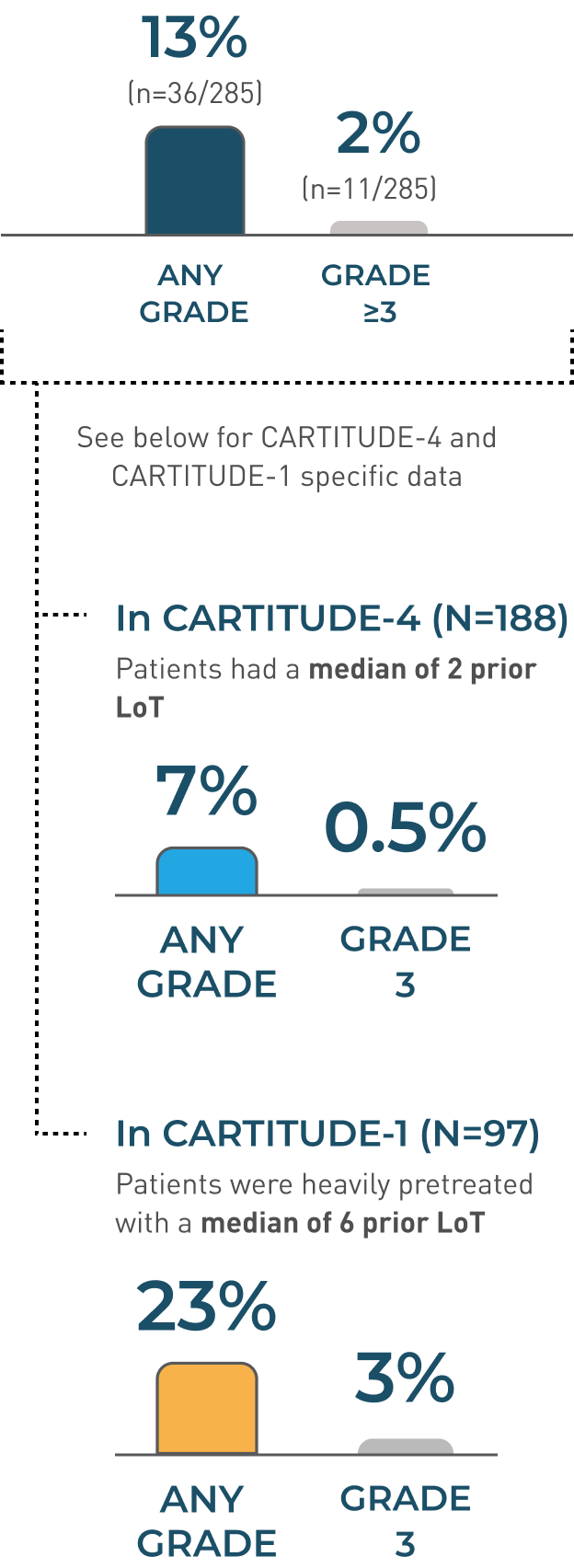
In CARTITUDE-4 and CARTITUDE-1 (N=285)1
Patients receiving CARVYKTI® may experience fatal or life-threatening ICANS following treatment with CARVYKTI®, including before CRS onset, concurrently with CRS, after CRS resolution, or in the absence of CRS
ICANS Incidence Rates

CARTITUDE-4 and CARTITUDE-1
POOLED DATA (N=285)

Additional ICANS Pooled Data
MEDIAN TIME TO ONSET
8 DAYS(range: 1-28 days)
MEDIAN DURATION
6 DAYS(range: 1-1229 days)
MEDIAN TIME TO RESOLUTION
3 DAYS(range: 1-143 days)
ICANS RESOLVED IN 83%
(n=30/36)


ICANS RESOLVED IN 83%
(n=30/36)
MONITOR PATIENTS FOR SIGNS AND SYMPTOMS OF ICANS*
At least daily for7 DAYSafter infusion
At least daily for2 WEEKSafter infusion
- ICANS resolved in 30 of 36 (83%) of patients with a median time to resolution of 3 days (range: 1-143 days)
- Of patients with ICANS, 97% (35/36) had CRS. The onset of ICANS occurred during CRS in 69% of patients, before and after the onset of CRS in 14% of patients, respectively
- The most frequent (≥2%) manifestations of ICANS included encephalopathy (12%), aphasia (4%), headache (3%), motor dysfunction (3%), ataxia (2%), and sleep disorder (2%)
- Monitor patients at least daily for 7 days following CARVYKTI® infusion for signs and symptoms of ICANS.* Rule out other causes of ICANS symptoms. Monitor patients for signs or symptoms of ICANS for at least 2 weeks after infusion and treat promptly. Neurologic toxicity should be managed with supportive care and/or corticosteroids as needed
*See CARVYKTI® USPI Table 2 Guideline of management for ICANS.
Parkinsonism
Parkinsonism Incidence Rates
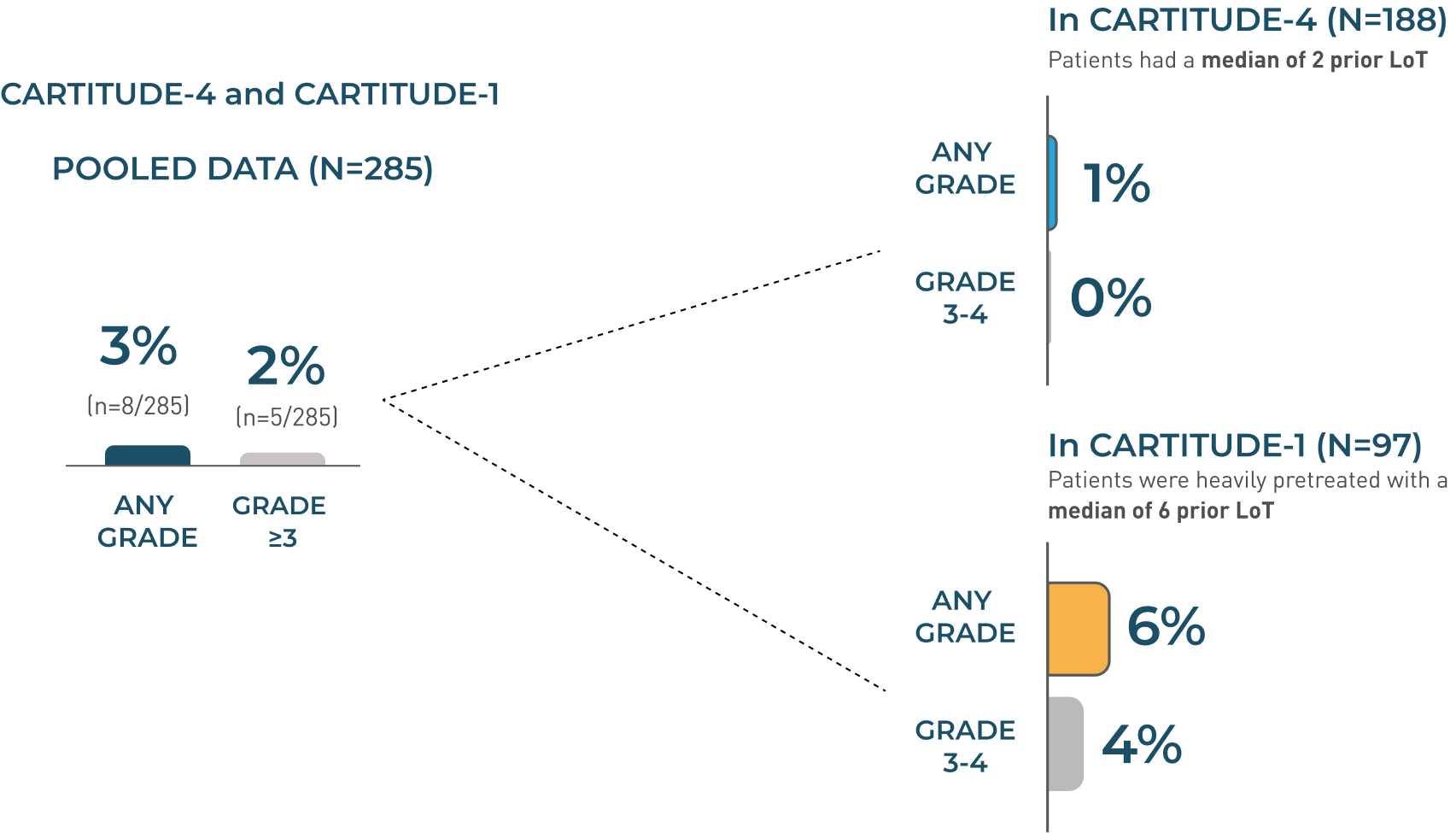
No new cases of parkinsonism were reported from the 27.7-month median follow-up (above) up to the 61.3-month median follow-up in CARTITUDE-1.2,3
Information is not included in the current USPI.
CARTITUDE-4 and CARTITUDE-1
POOLED DATA (N=285)
No new cases of parkinsonism were reported from the 27.7-month median follow-up (above) up to the 61.3-month median follow-up in CARTITUDE-1.2,3
Information is not included in the current USPI.
Additional Parkinsonism Pooled Data
MEDIAN TIME TO ONSET
56 DAYS(range: 14-914 days)
MEDIAN DURATION
243.5 daysrange: 62-720 days
MEDIAN TIME TO RESOLUTION
523 DAYS(range: 1-544 days)
Parkinsonism RESOLVED in 13%
(n=1/8)


Parkinsonism RESOLVED in 13%
(n=195/238)
- The manifestations of parkinsonism included movement disorders, cognitive impairment, and personality changes
- Monitor patients for signs and symptoms of parkinsonism that may be delayed in onset and managed with supportive care measures. There is limited efficacy information with medications used for the treatment of Parkinson’s disease for the improvement or resolution of parkinsonism symptoms following CARVYKTI® treatment
- In CARTITUDE-4, 1% experienced any-grade parkinsonism, and no patients experienced Grade 3 or 4 parkinsonism
Guillain-Barré syndrome (GBS)
- A fatal outcome following GBS occurred following treatment with CARVYKTI® despite treatment with intravenous immunoglobulins
- Symptoms reported include those consistent with Miller-Fisher variant of GBS, encephalopathy, motor weakness, speech disturbances, and polyradiculoneuritis
- Monitor for GBS. Evaluate patients presenting with peripheral neuropathy for GBS
- Consider treatment of GBS with supportive care measures and in conjunction with immunoglobulins and plasma exchange, depending on severity of GBS
Immune-mediated myelitis
- Grade 3 myelitis occurred 25 days following treatment with CARVYKTI® in CARTITUDE-4 in a patient who received CARVYKTI® as subsequent therapy
- Symptoms reported included hypoesthesia of the lower extremities and the lower abdomen with impaired sphincter control
- Symptoms improved with the use of corticosteroids and intravenous immune globulin. Myelitis was ongoing at the time of death from other cause
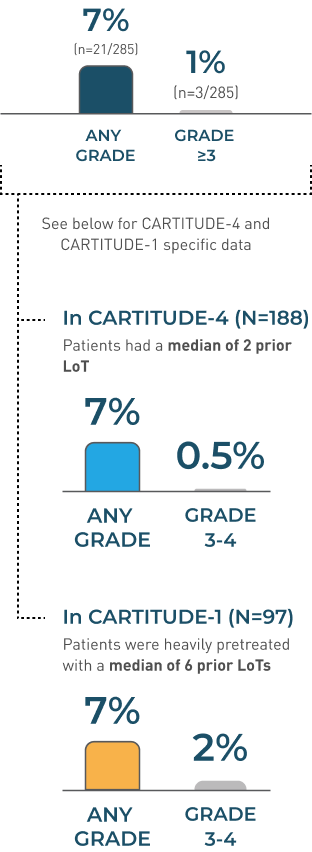
Peripheral neuropathy
peripheral neuropathy Incidence Rates
CARTITUDE-4 and CARTITUDE-1
POOLED DATA (N=285)

ADDITIONAL peripheral neuropathy POOLED DATA
MEDIAN TIME TO ONSET
57 DAYS(range: 1-914 days)
MEDIAN DURATION
149.5 days†(range: 1-692 days)
MEDIAN TIME TO RESOLUTION
58 DAYS(range: 1-215 days)
peripheral neuropathy RESOLVED in 52%
(n=11/21)


peripheral neuropathy RESOLVED IN 52%
(n=11/21)
- Monitor patients for signs and symptoms of peripheral neuropathies
- Patients who experience peripheral neuropathy may also experience cranial nerve palsies or GBS
†Including patients with ongoing neurologic events at the time of death or data cut-off.
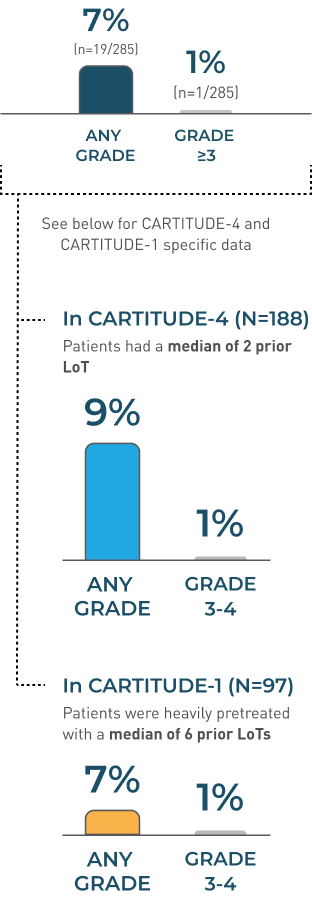
Cranial nerve palsies
CRANIAL NERVE PALSIES Incidence Rates

No new cases of cranial nerve palsies were reported from the 27.7-month median follow-up (above) up to the 61.3-month median follow-up in CARTITUDE-1.2,3
Information is not included in the current USPI.
CARTITUDE-4 and CARTITUDE-1
POOLED DATA (N=285)
No new cases of cranial nerve palsies were reported from the 27.7-month median follow-up (above) up to the 61.3-month median follow-up in CARTITUDE-1.2,3
Information is not included in the current USPI.
ADDITIONAL CRANIAL NERVE PALSIES POOLED DATA
MEDIAN TIME TO ONSET
21 DAYS(range: 17-101 days)
MEDIAN DURATION
70 DAYS (range: 1-262 days)
MEDIAN TIME TO RESOLUTION
66 DAYS(range: 1-209 days)
Cranial Nerve Palsies Resolved in 89%
(n=17/19)


Cranial Nerve Palsies Resolved in 89%
(n=17/19)
- The most frequent cranial nerve affected was the 7th cranial nerve. Additionally, cranial nerves III, V, and VI have been reported to be affected
- Monitor patients for signs and symptoms of cranial nerve palsies. Consider management with systemic corticosteroids, depending on the severity and progression of signs and symptoms
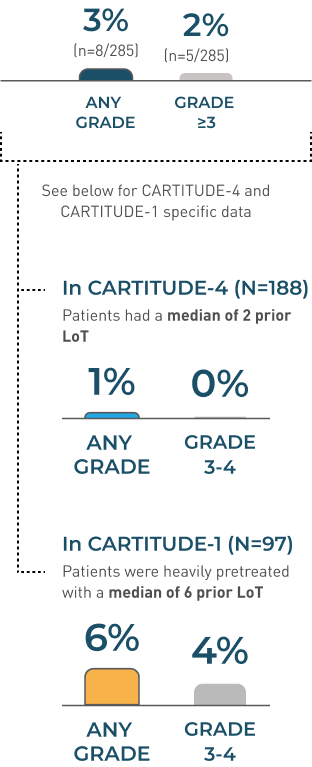
Hemophagocytic Lymphohistiocytosis/
Macrophage Activation Syndrome (HLH/MAS)
In CARTITUDE-4 and CARTITUDE-1 (N=285)1
HLH/MAS is a potentially life-threatening complication
| Any grade |
|---|
| 1%(n=3/285) |
MEDIAN TIME TO ONSET
10 DAYS
(range 8-99 days)
- All events of HLH/MAS occurred in the setting of ongoing or worsening CRS
- The manifestations of HLH/MAS included hyperferritinemia, hypotension, hypoxia with diffuse alveolar damage, coagulopathy and hemorrhage, cytopenia and multi-organ dysfunction, including renal dysfunction and respiratory failure
- Patients who develop HLH/MAS have an increased risk of severe bleeding. Monitor hematologic parameters in patients with HLH/MAS and transfuse per institutional guidelines. Fatal cases of HLH/MAS occurred following treatment with CARVYKTI®
- HLH is a life-threatening condition with a high mortality rate if not recognized and treated early. Treatment of HLH/MAS should be administered per institutional standards
Prolonged and/or Recurrent Cytopenias
In CARTITUDE-4 and CARTITUDE-1 (N=285)1
Patients may exhibit prolonged and recurrent cytopenias following lymphodepleting chemotherapy and CARVYKTI® infusion
| PERCENT OF PATIENTS WITH GRADE ≥3 CYTOPENIAS THAT DID NOT RESOLVE BY DAY 30 FOLLOWING CARVYKTI® INFUSION (n/N) | |
|---|---|
Overall 62% (176/285) | |
thrombocytopenia 33% (94/285) | neutropenia 27% (76/285) |
lymphopenia 24% (67/285) | anemia 2% (6/285) |
| PERCENT OF PATIENTS WITH RECURRENCE OF GRADE 3 OR 4 CYTOPENIA AFTER INITIAL RECOVERY | |
|---|---|
Overall 77% (219/285) | |
thrombocytopenia 5% | neutropenia 20% |
lymphopenia 22% | anemia 6% |
| Overall | thrombocytopenia | neutropenia | lymphopenia | anemia | |
|---|---|---|---|---|---|
| PERCENT OF PATIENTS WITH GRADE ≥3 CYTOPENIAS THAT DID NOT RESOLVE BY DAY 30 FOLLOWING CARVYKTI® INFUSION (n/N) | 62% (176/285) | 33% (94/285) | 27% (76/285) | 24% (67/285) | 2% (6/285) |
| PERCENT OF PATIENTS WITH RECURRENCE OF GRADE 3 OR 4 CYTOPENIA AFTER INITIAL RECOVERY | 77% (219/285) ≥1 recurrence | 5% | 20% | 22% | 6% |
AFTER DAY 60 FOLLOWING CARVYKTI® INFUSION
- Seventy-seven percent (219/285) of patients had one, two, or three or more recurrences of Grade 3 or 4 cytopenias after initial recovery of Grade 3 or 4 cytopenia
- Sixteen and 25 patients had Grade 3 or 4 neutropenia and thrombocytopenia, respectively, at the time of death
- Monitor blood counts prior to and after CARVYKTI® infusion. Manage cytopenias with growth factors and blood product transfusion support according to local institutional guidelines
CARTITUDE-4
Grade 3 or 4 laboratory abnormalities in ≥10% of patients treated with CARVYKTI® (N=188) and standard therapy (N=208)
| Laboratory abnormality | CARVYKTI® (N=188) Grade 3 or 4 (%) |
|---|---|
| LYMPHOCYTE COUNT DECREASED | 99 |
| NEUTROPHIL COUNT DECREASED | 95 |
| White BLOOD CELLS DECREASED | 94 |
| PLATELET COUNT DECREASED | 47 |
| HEMOGLOBIN DECREASED | 34 |
| Laboratory abnormality | Standard Therapy (N=208) Grade 3 or 4 (%) |
| LYMPHOCYTE COUNT DECREASED | 62 |
| NEUTROPHIL COUNT DECREASED | 88 |
| White BLOOD CELLS DECREASED | 69 |
| PLATELET COUNT DECREASED | 20 |
| HEMOGLOBIN DECREASED | 17 |
| Laboratory abnormality | CARVYKTI® | Standard Therapy |
|---|---|---|
| LYMPHOCYTE COUNT DECREASED | 99 | 62 |
| NEUTROPHIL COUNT DECREASED | 95 | 88 |
| White BLOOD CELL DECREASED | 94 | 69 |
| PLATELET COUNT DECREASED | 47 | 20 |
| HEMOGLOBIN DECREASED | 34 | 17 |
CARTITUDE-1
Grade 3 or 4 laboratory abnormalities in ≥10% of patients treated with CARVYKTI® (N=97)
| Laboratory abnormality | Grade 3-4 (%) |
|---|---|
| Lymphopenia | 99 |
| Neutropenia | 98 |
| White blood cells decreased | 98 |
| ANEMIA | 72 |
| THROMBOCYTOPENIA | 63 |
| ASPARTATE AMINOTRANSFERASE INCREASED | 21 |
IMMUNE EFFECTOR CELL-ASSOCIATED
ENTEROCOLITIS (IEC-EC)1
- Manifestations include severe or prolonged diarrhea, abdominal pain, and weight loss requiring total parenteral nutrition
- IEC-EC has been associated with fatal outcome from perforation or sepsis
- Manage according to institutional guidelines, including referral to gastroenterology and infectious disease specialists
- In cases of refractory IEC-EC, consider additional workup to exclude alternative etiologies, including T-cell lymphoma of the GI tract, which has been reported in the postmarketing setting
Secondary malignancies
In CARTITUDE-4 and CARTITUDE-1 (N=285)1
Patients treated with CARVYKTI® may develop secondary malignancies
- Myeloid neoplasms occurred in 5% (13/285) of patients (9 cases of myelodysplastic syndrome, 3 cases of acute myeloid leukemia, and 1 case of myelodysplastic syndrome followed by acute myeloid leukemia)
- The median time to onset of myeloid neoplasms was 447 days (range: 56 to 870 days) after treatment with CARVYKTI®
- Ten of these 13 patients died following the development of myeloid neoplasms; 2 of the 13 cases of myeloid neoplasm occurred after initiation of subsequent antimyeloma therapy
- Cases of myelodysplastic syndrome and acute myeloid leukemia have also been reported in the post marketing setting
- T-cell malignancies have occurred following treatment of hematologic malignancies with BCMA and CD19-directed genetically modified autologous T-cell immunotherapies, including CARVYKTI®. Mature T-cell malignancies, including CAR-positive tumors, may present as soon as weeks following infusions, and may include fatal outcomes
- Monitor life-long for secondary malignancies. In the event that a secondary malignancy occurs, contact Janssen Biotech, Inc., at 1-800-526-7736 for reporting and to obtain instructions on collection of patient samples
Increased Early Mortality
In CARTITUDE-4 (N=208)1*
- There was a numerically higher percentage of early deaths in patients randomized to the CARVYKTI® treatment arm compared to the control arm
- Among patients with deaths occurring within the first 10 months from randomization, a greater proportion (29/208; 14%) occurred in the CARVYKTI® arm compared to (25/211; 12%) in the control arm
- Of the 29 deaths that occurred in the CARVYKTI® arm within the first 10 months of randomization, 10 deaths occurred prior to CARVYKTI® infusion, and 19 deaths occurred after CARVYKTI® infusion
- Of the 10 deaths that occurred prior to CARVYKTI® infusion, all occurred due to disease progression, and none occurred due to adverse events
- Of the 19 deaths that occurred after CARVYKTI® infusion, 3 occurred due to disease progression, and 16 occurred due to adverse events
- The most common adverse events were due to infection (n=12)
- Inform patients of the risk of early mortality
*Evaluated in the intent-to-treat population (419 patients randomized to receive CARVYKTI® (N=208) or standard therapy (N=211)).
INFECTIONS
In CARTITUDE-1 and CARTITUDE-4 (N=285)1
- CARVYKTI® should not be administered to patients with active infection or inflammatory disorders. Severe, life-threatening, or fatal infections occurred in patients after CARVYKTI® infusion
- Infections occurred in 57% (163/285), including ≥Grade 3 in 24% (69/285) of patients
- Grade 3 or 4 infections with an unspecified pathogen occurred in 12%, viral infections in 6%, bacterial infections in 5%, and fungal infections in 1% of patients
- Overall, 5% (13/285) of patients had Grade 5 infections, 2.5% of which were due to COVID-19
- Patients treated with CARVYKTI® had an increased rate of fatal COVID-19 infections compared to the standard therapy arm
- Monitor patients for signs and symptoms of infection before and after CARVYKTI® infusion and treat patients appropriately
- Administer prophylactic, pre-emptive, and/or therapeutic antimicrobials according to the standard institutional guidelines
- Febrile neutropenia was observed in 5% of patients after CARVYKTI® infusion and may be concurrent with CRS. In the event of febrile neutropenia, evaluate for infection and manage with broad-spectrum antibiotics, fluids, and other supportive care as medically indicated
- Counsel patients on the importance of prevention measures
- Follow institutional guidelines for the vaccination and management of immunocompromised patients with COVID-19
- Viral Reactivation: Hepatitis B virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure, and death, can occur in patients with hypogammaglobulinemia. Perform screening for Cytomegalovirus (CMV), HBV, hepatitis C virus (HCV), and human immunodeficiency virus (HIV) or any other infectious agents if clinically indicated in accordance with clinical guidelines before collection of cells for manufacturing
- Consider antiviral therapy to prevent viral reactivation per local institutional guidelines/clinical practice
- Reactivation of John Cunningham (JC) virus, leading to progressive multifocal leukoencephalopathy (PML), including cases with fatal outcomes, have been reported following treatment
- Perform appropriate diagnostic evaluations in patients with neurological adverse events
HYPOGAMMAGLOBULINEMIA
In CARTITUDE-1 and CARTITUDE-4 (N=285)1
- Hypogammaglobulinemia adverse event was reported in 36% (102/285) of patients; laboratory IgG levels fell below 500 mg/dL after infusion in 93% (265/285) of patients
- Hypogammaglobulinemia either as an adverse reaction or laboratory IgG level below 500 mg/dL after infusion occurred in 94% (267/285) of patients treated
- Fifty-six percent (161/285) of patients received intravenous immunoglobulin (IVIG) post CARVYKTI® for either an adverse reaction or prophylaxis
- Monitor immunoglobulin levels after treatment with CARVYKTI® and administer IVIG for IgG <400 mg/dL. Manage per local institutional guidelines, including infection precautions and antibiotic or antiviral prophylaxis
- Use of Live Vaccines: The safety of immunization with live viral vaccines during or following CARVYKTI® treatment has not been studied
- Vaccination with live virus vaccines is not recommended for at least 6 weeks prior to the start of lymphodepleting chemotherapy, during CARVYKTI® treatment, and until immune recovery following treatment with CARVYKTI®
HYPERSENSITIVITY REACTIONS
In CARTITUDE-1 and CARTITUDE-4 (N=285)1
- Hypersensitivity reactions occurred in 5% (13/285), all of which were ≤Grade 2
- Manifestations of hypersensitivity reactions included flushing, chest discomfort, tachycardia, wheezing, tremor, burning sensation, non-cardiac chest pain, and pyrexia
- Serious hypersensitivity reactions, including anaphylaxis, may be due to the dimethyl sulfoxide (DMSO) in CARVYKTI®
- Patients should be carefully monitored for 2 hours after infusion for signs and symptoms of severe reaction
- Treat promptly and manage patients appropriately according to the severity of the hypersensitivity reaction
IMMUNE EFFECTOR CELL-ASSOCIATED
ENTEROCOLITIS (IEC-EC)
- Manifestations include severe or prolonged diarrhea, abdominal pain and weight loss requiring total parenteral nutrition
- IEC-EC has been associated with fatal outcome from perforation or sepsis
- Manage according to institutional guidelines, including referral to gastroenterology and infectious disease specialists
- In cases of refractory IEC-EC, consider additional workup to exclude alternative etiologies, including T-cell lymphoma of the GI tract, which has been reported in the postmarketing setting
Adverse Reactions ≥10%
In CARTITUDE-41
| ADVERSE REACTIONS OBSERVED IN AT LEAST 10% OF PATIENTS TREATED WITH CARVYKTI® AND STANDARD THERAPY IN CARTITUDE-4 STUDY | ||||
| CARVYKTI® N=188 | CARVYKTI® N=188 | Standard Therapy N=208 | Standard Therapy N=208 | |
|---|---|---|---|---|
| CARVYKTI® N=188 | Standard Therapy N=208 | |||
| System Organ Class (SOC) Preferred term | Any Grade (%) | Grade 3 or Higher (%) | Any Grade (%) | Grade 3 or Higher (%) |
| Gastrointestinal disorders | ||||
| Diarrhea† | 27 | 3 | 27 | 2 |
| Nausea | 20 | 0 | 18 | 1 |
| Constipation | 10 | 0 | 21 | 1 |
| General disorders and administrative site conditions | ||||
| Pyrexia | 79 | 5 | 16 | 1 |
| Fatigue* | 28 | 3 | 50 | 3 |
| Edema‡ | 11 | 1 | 20 | 1 |
| Pain* | 10 | 1 | 14 | <1 |
| Immune system disorders | ||||
| Hypogammaglobulinemia§ | 94 | 9 | 72 | <1 |
| Cytokine release syndrome | 78 | 3 | <1 | 0 |
| INFECTIONS AND INFESTATIONS | ||||
| Upper respiratory tract infection* | 25 | 1 | 40 | 5 |
| Viral infection* | 23 | 4 | 31 | 6 |
| Bacterial infections* | 15 | 6 | 17 | 4 |
| Pneumonia* | 14 | 9 | 18 | 11 |
| Metabolism and nutrition disorders | ||||
| Decreased appetite | 10 | 0 | 5 | 0 |
| Musculoskeletal and connective tissue disorders | ||||
| Musculoskeletal pain* | 34 | 2 | 47 | 4 |
| Nervous system disorders | ||||
| Headache* | 23 | 0 | 13 | 0 |
| Encephalopathy|| | 11 | 2 | 4 | 1 |
| RESPIRATORY, THORACIC, AND MEDIASTINAL DISORDERS | ||||
| Cough* | 15 | 0 | 18 | 0 |
| Hypoxia | 12 | 3 | 1 | 1 |
| Vascular Disorders | ||||
| Hypotension* | 23 | 4 | 3 | 0 |
*Represents multiple related terms.
†Diarrhea includes Colitis, and Diarrhea.
‡Edema includes Face edema, Generalized edema, Localized edema, Edema peripheral, Periorbital edema, Peripheral swelling, Pulmonary edema, and Scrotal edema.
§Hypogammaglobulinemia includes subjects with adverse event of hypogammaglobulinemia and/or laboratory IgG levels that fell below 500 mg/dL following CARVYKTI® infusion or standard therapy.
‖Encephalopathy includes Amnesia, Bradyphrenia, Confusional state, Depressed level of consciousness, Disturbance in attention, Immune effector cell-associated neurotoxicity syndrome, Lethargy, and Psychomotor retardation.
Adverse Reactions ≥10%
In CARTITUDE-11
| Adverse reactions observed in at least 10% of patients treated with CARVYKTI® in CARTITUDE-1 (N=97) | ||
| System Organ Class (SOC) Preferred Term | Any Grade (%) | Grade 3 or Higher (%) |
|---|---|---|
| Blood and lymphatic system disorders | ||
| Coagulopathy† | 22 | 2 |
| Febrile neutropenia | 10 | 9 |
| Cardiac Disorders | ||
| Tachycardia* | 27 | 1 |
| Gastrointestinal Disorders | ||
| Diarrhea‡ | 33 | 1 |
| Nausea | 31 | 1 |
| Constipation | 22 | 0 |
| Vomiting | 20 | 0 |
| GENERAL DISORDERS AND ADMINISTRATIVE SITE CONDITIONS | ||
| Pyrexia | 96 | 5 |
| Fatigue* | 47 | 7 |
| Chills | 33 | 0 |
| Edema§ | 23 | 0 |
| Immune system disorders | ||
| Cytokine release syndrome* | 95 | 5 |
| Hypogammaglobulinemia|| | 93 | 2 |
| INFECTIONS AND INFESTATIONS | ||
| Infections-pathogen unspecified* | 41 | 19 |
| Upper respiratory tract infection* | 28 | 3 |
| Viral infections* | 23 | 7 |
| Pneumonia* | 14 | 13 |
| Sepsis* | 10 | 7 |
| Metabolism and nutrition disorders | ||
| Decreased appetite | 29 | 1 |
| Musculoskeletal and connective tissue disorders | ||
| Musculoskeletal pain* | 48 | 2 |
| Nervous system disorders | ||
| Encephalopathy¶ | 30 | 6 |
| Headache | 27 | 0 |
| Dizziness* | 23 | 1 |
| Motor dysfunction# | 16 | 3 |
| Psychiatric disorders | ||
| Insomnia | 13 | 0 |
| RESPIRATORY, THORACIC, AND MEDIASTINAL DISORDERS | ||
| Cough* | 39 | 0 |
| Dyspnea** | 23 | 3 |
| Nasal congestion | 15 | 0 |
| Hypoxia | 12 | 4 |
| NEOPLASMS: BENIGN, MALIGNANT, AND UNSPECIFIED (INCL CYSTS AND POLYPS) | ||
| Hematologic malignancy†† | 10 | 10 |
| Vascular Disorders | ||
| Hypotension* | 51 | 10 |
| Hypertension | 19 | 6 |
| Hemorrhage‡‡ | 16 | 4 |
*Represents multiple related terms.
†Coagulopathy includes Activated partial thromboplastin time prolonged, Disseminated intravascular coagulation, Hypofibrinogenemia, International normalized ratio increased, and Prothrombin time prolonged.
‡Diarrhea includes Colitis and Diarrhea.
§Edema includes Face edema, Generalized edema, Localized edema, Edema peripheral, Periorbital edema, Peripheral swelling, Pulmonary edema, and Scrotal edema.
‖Hypogammaglobulinemia includes subjects with adverse event of hypogammaglobulinemia and/or laboratory IgG levels that fell below 500 mg/dL following CARVYKTI® infusion.
¶Encephalopathy includes Amnesia, Bradyphrenia, Confusional state, Depressed level of consciousness, Disturbance in attention, Immune effector cell–associated neurotoxicity syndrome, Lethargy, Memory impairment, Mental impairment, Mental status changes, Noninfective encephalitis, and Somnolence.
# Motor dysfunction includes Muscle spasms, Muscle tightness, Muscular weakness, and Myoclonus.
** Dyspnea includes Acute respiratory failure, Dyspnea exertional, Respiratory failure, and Tachypnea.
††Hematologic malignancy includes Myelodysplastic syndrome and Acute myeloid leukemia.
‡‡Hemorrhage includes Conjunctival hemorrhage, Contusion, Ecchymosis, Epistaxis, Eye contusion, Hematochezia, Hemoptysis, Infusion site hematoma, Oral contusion, Petechiae, Post procedural hemorrhage, Pulmonary hemorrhage, Retinal hemorrhage, and Subdural hematoma.
LONG-TERM SAFETY PROFILE
CARTITUDE-4 median follow-up of 33.6 months
You are now viewing a subsequent follow-up analysis from the CARTITUDE-4 trial. This information is not included in the current USPI.
The safety data described in this section reflect the CARTITUDE-4 follow-up safety analysis at 33.6 months. The safety population of CARVYKTI® includes
all patients who were randomized to CARVYKTI® (N=208)1
The safety data described in this section reflect the CARTITUDE-4 follow-up safety analysis at 33.6 months. The safety population of CARVYKTI® includes all patients who were randomized to CARVYKTI® (N=208)1
| INFECTIONS | CARVYKTI® (N=208) | Standard Therapy (N=208) |
|---|---|---|
| Treatment-emergent infections, (%) | ||
| All Grade | 63.5 | 76.4 |
| Grade 3/4 | 28.4 | 29.8 |
| Deaths due to TE- and non-TE infections, n | 16 | 19 |
| In first year, n | 13 | 8 |
| In second year, n | 2 | 8 |
| INFECTIONS | CARVYKTI® (N=208) | |
|---|---|---|
| Treatment-emergent infections, (%) | ||
| All Grade | 63.5 | |
| Grade 3/4 | 28.4 | |
| Deaths due to TE- and non-TE infections, n | 16 | |
| In first year, n | 13 | |
| In second year, n | 2 | |
| INFECTIONS | Standard Therapy (N=208) | |
|---|---|---|
| Treatment-emergent infections, (%) | ||
| All Grade | 76.4 | |
| Grade 3/4 | 29.8 | |
| Deaths due to TE- and non-TE infections, n | 19 | |
| In first year, n | 8 | |
| In second year, n | 8 | |
| CAUSE OF DEATH | CARVYKTI® (N=208) | Standard Therapy (N=208) |
|---|---|---|
| Deaths, n | 50 | 82 |
| Due to progressive disease | 21 | 51 |
| Due to TEAE | 12 | 8 |
| CAUSE OF DEATH | CARVYKTI® (N=208) |
|---|---|
| Deaths, n | 50 |
| Due to progressive disease | 21 |
| Due to TEAE | 12 |
| CAUSE OF DEATH | Standard Therapy (N=208) |
|---|---|
| Deaths, n | 82 |
| Due to progressive disease | 51 |
| Due to TEAE | 8 |
| SECONDARY PRIMARY MALIGNANCIES | CARVYKTI® (N=208) | Standard Therapy (N=208) |
|---|---|---|
| SPMs, n (%) | 27 (13.0) | 24 (11.5) |
| Hematologic,* n (%) | 7 (3.4) | 1 (0.5) |
| MDS, n | 4 | 0 |
| Progresses to AML, n | 2 | - |
| AML, n | 1 | 0 |
| Peripheral T-cell lymphoma, n | 2 | 0 |
| EBV-associated lymphoma, n | 0 | 1 |
| Cutaneous/non-invasive* | 15 (7.2) | 15 (7.2) |
| Non-cutaneous/invasive* | 6 (2.9) | 8 (3.8) |
| SECONDARY PRIMARY MALIGNANCIES | CARVYKTI® (N=208) |
|---|---|
| SPMs, n (%) | 27 (13.0) |
| Hematologic,* n (%) | 7 (3.4) |
| MDS, n | 4 |
| Progresses to AML, n | 2 |
| AML, n | 1 |
| Peripheral T-cell lymphoma, n | 2 |
| EBV-associated lymphoma, n | 0 |
| Cutaneous/non-invasive* | 15 (7.2) |
| Non-cutaneous/ invasive* | 6 (2.9) |
| SECONDARY PRIMARY MALIGNANCIES | Standard Therapy (N=208) |
|---|---|
| SPMs, n (%) | 24 (11.5) |
| Hematologic,* n (%) | 1 (0.5) |
| MDS, n | 0 |
| Progresses to AML, n | - |
| AML, n | 0 |
| Peripheral T-cell lymphoma, n | 0 |
| EBV-associated lymphoma, n | 1 |
| Cutaneous/non-invasive* | 15 (7.2) |
| Non-cutaneous/ invasive* | 8 (3.8) |
- Both arms had Grade 3/4 TEAE around 97%; most frequently cytopenia
- There have been no additional incidences of cranial nerve palsy or MNT since the previous data cutoff, as reported by San-Miguel J, et al.
in the NEJM 15.9-month follow-up (see reference 2), whether patients received CARVYKTI® as study treatment (n=176) or received CARVYKTI® as subsequent therapy2- Among the patients who received CARVYKTI® as a study treatment (n=176) in CARTITUDE-4, there were a total of 16 cases of CNP, of which 14 cases recovered, and 1 case of ongoing MNT2
AML=acute myeloid leukemia; CNP=cranial nerve palsy; EBV=Epstein-Barr virus; MDS=myelodysplastic syndrome; MNT=movement and neurocognitive treatment-emergent adverse event; SPM=second primary malignancy; TE=treatment emergent; TEAE=treatment-emergent adverse event; USPI=US Prescribing Information.
*Multiple SPMs could occur in the same patient.