PROGRESSION-FREE SURVIVAL
In CARTITUDE-4 at 33.6 Months*
the current USPI and should be interpreted with caution. The data are presented here for descriptive purposes only.
PROGRESSION-FREE SURVIVAL1-4
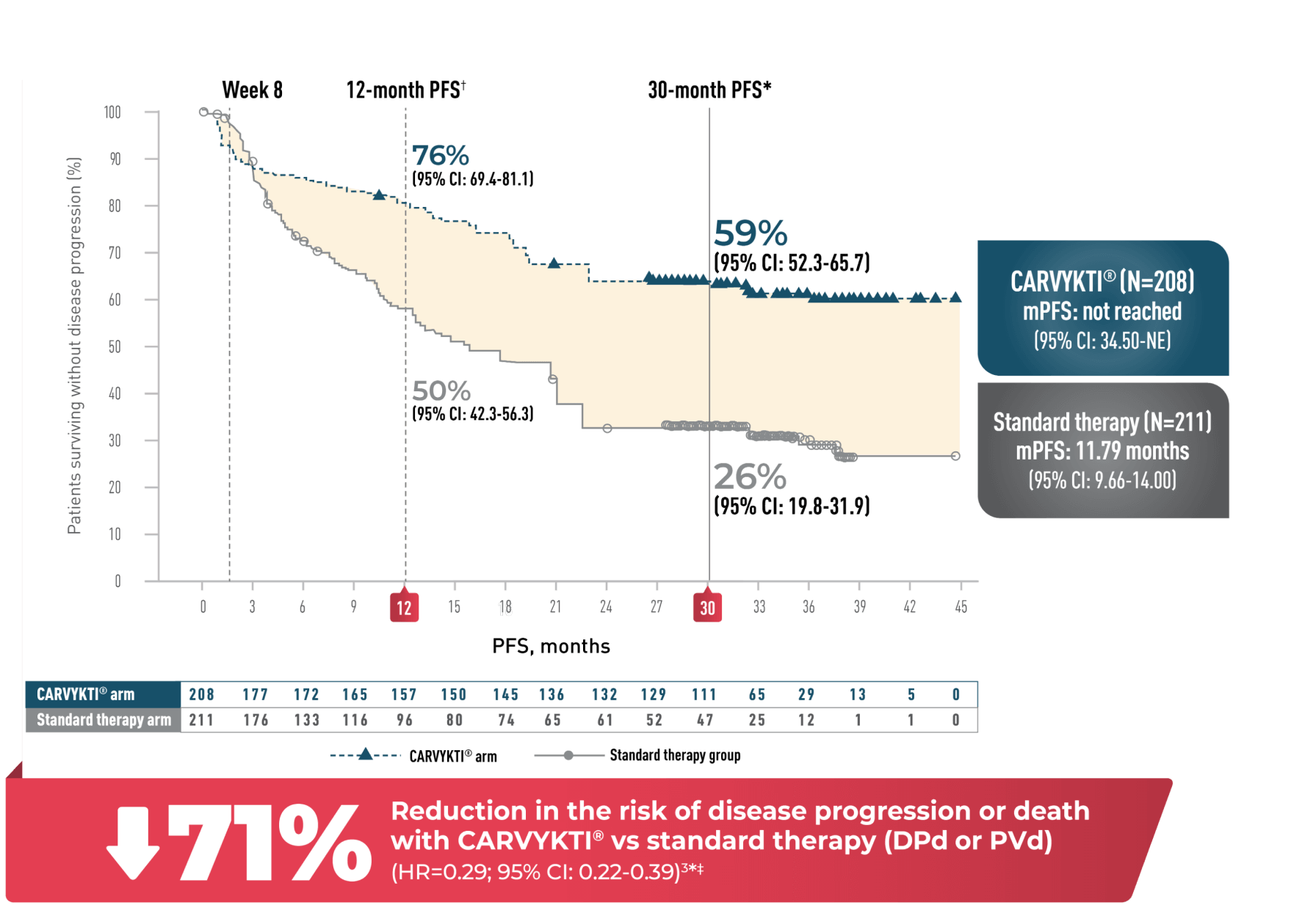
Percentages rounded to nearest whole number. This data is calculated from a weighted analysis.
CI=confidence interval; DPd=daratumumab, pomalidomide, and dexamethasone; FDA=U.S. Food and Drug Administration; HR=hazard ratio; mPFS=median progression-free survival; NE=not estimable; PFS=progression-free survival; PVd=pomalidomide, bortezomib, and dexamethasone; USPI=US Prescribing Information.
*Median follow-up was 33.6 months in the Intent-to-Treat Analysis Set.
†12-month PFS values are derived from the USPI and are based on independent review committee (IRC) assessment of progression, FDA-requested analysis approach for PFS, and the 01 November 2022 data cutoff.
‡HR and 95% CI from a Cox proportional hazards model with treatment as the sole explanatory variable, including only PFS events that occurred
>8 weeks post randomization.
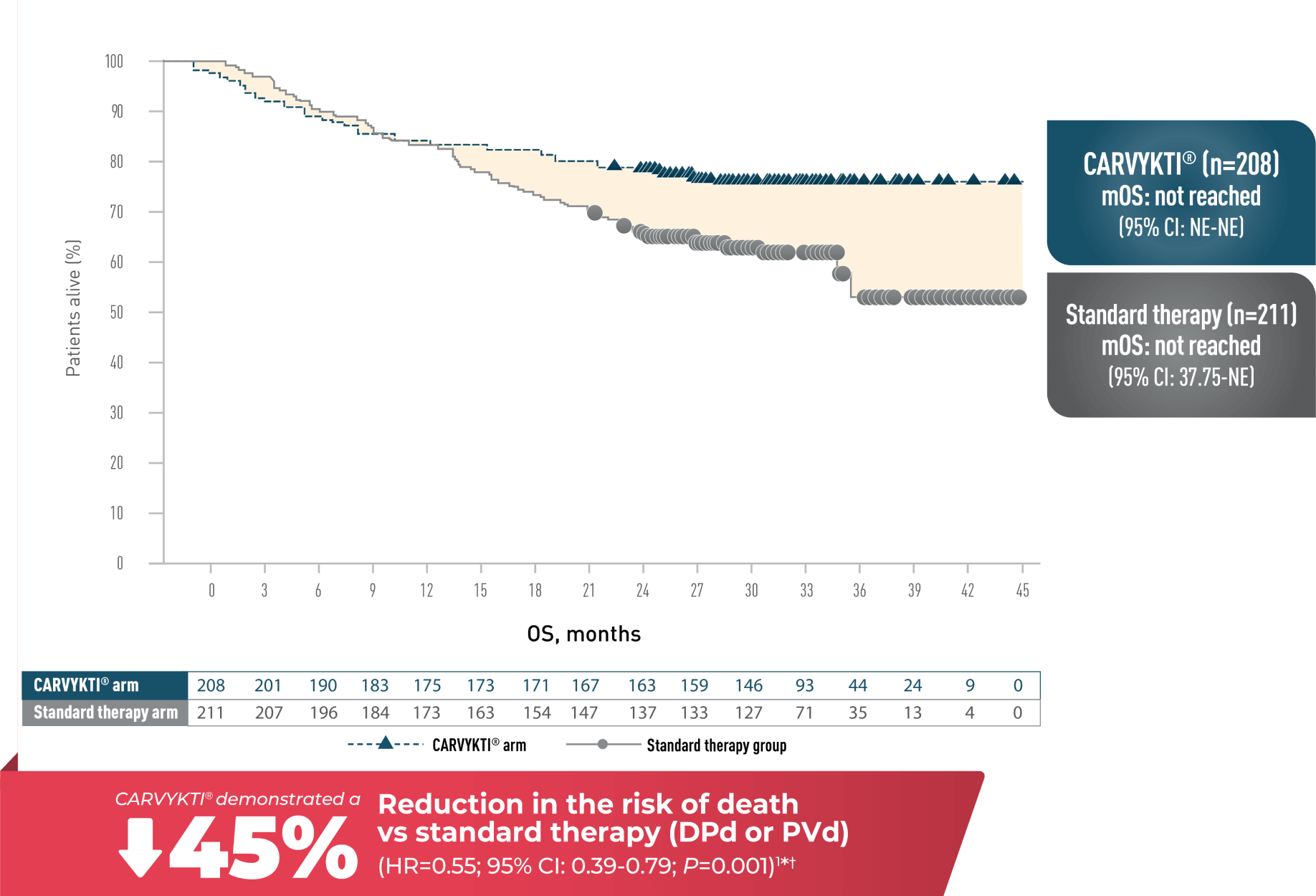
OVERALL SURVIVAL WITH A MEDIAN FOLLOW-UP OF 33.6 MONTHS
CARVYKTI® DEMONSTRATED A STATISTICALLY SIGNIFICANT OVERALL SURVIVAL BENEFIT IN 2L+1
CARTITUDE-4 median follow-up of 33.6 months
OVERALL SURVIVAL1*†
ESTIMATED OS RATE AT 12 MONTHS AND 30 MONTHS
CARTITUDE-4 median follow-up of 33.6 months
survival at 12 and 30 months is not included in the USPI and should be interpreted with
caution. The data are presented here for descriptive purposes only.
current USPI and should be
interpreted with caution. The data are presented here for descriptive
purposes only.
OVERALL SURVIVAL1-4*†
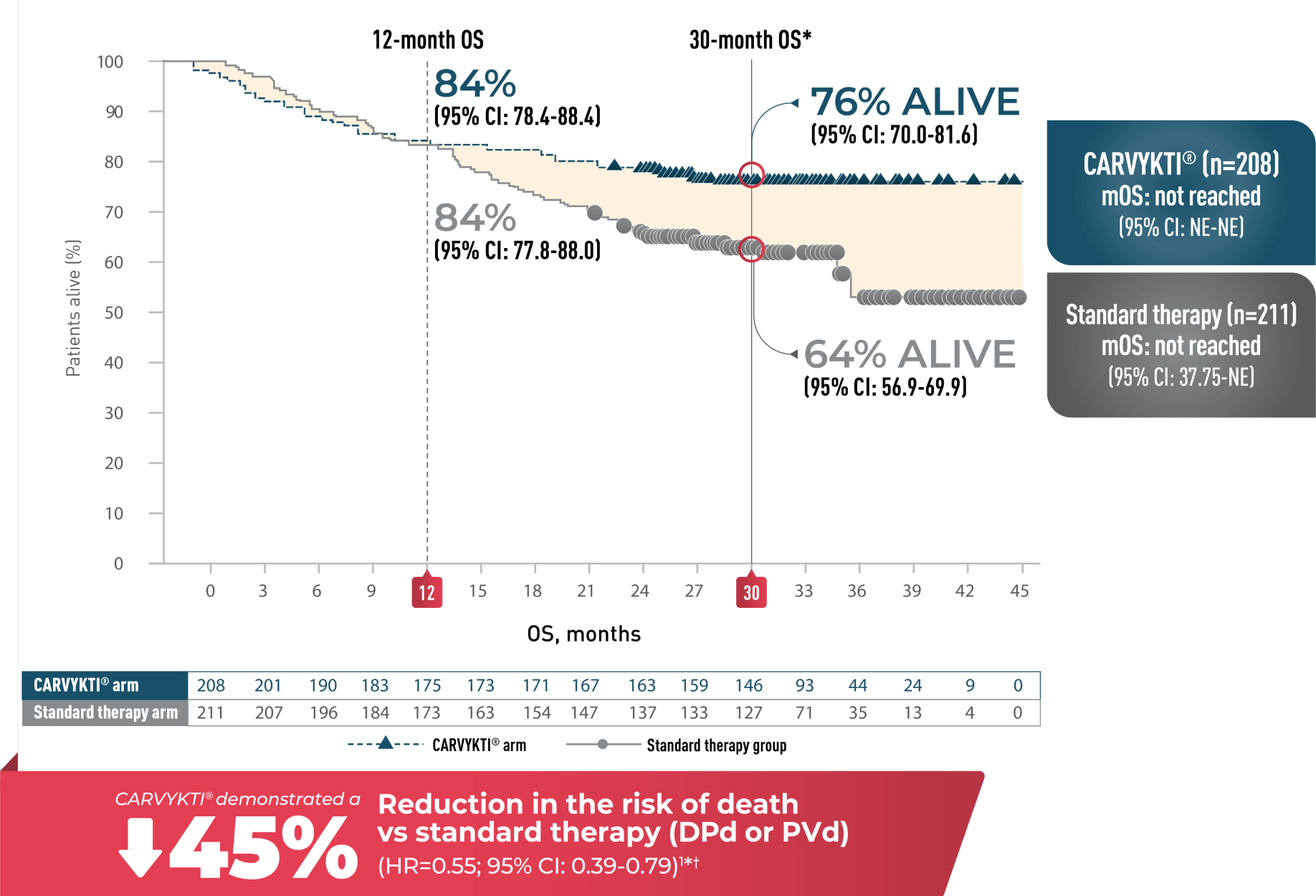
Percentages rounded to nearest whole number.
2L=second line; CI=confidence interval; DPd=daratumumab, pomalidomide, and dexamethasone; HR=hazard ratio;
mOS=median overall survival; NE=not estimable; OS=overall survival; PVd=pomalidomide, bortezomib, and dexamethasone;
USPI=US Prescribing Information.
*Median follow-up was 33.6 months in the Intent-to-Treat Analysis Set.
†Hazard ratio and 95% CI from a Cox proportional hazards model with treatment as the sole explanatory variable.
OVERALL RESPONSE RATE
In CARTITUDE-4 at 33.6 Months*†
the current USPI and should be interpreted with caution. The data are presented here for descriptive purposes only.
OVERALL RESPONSE RATE1,2*
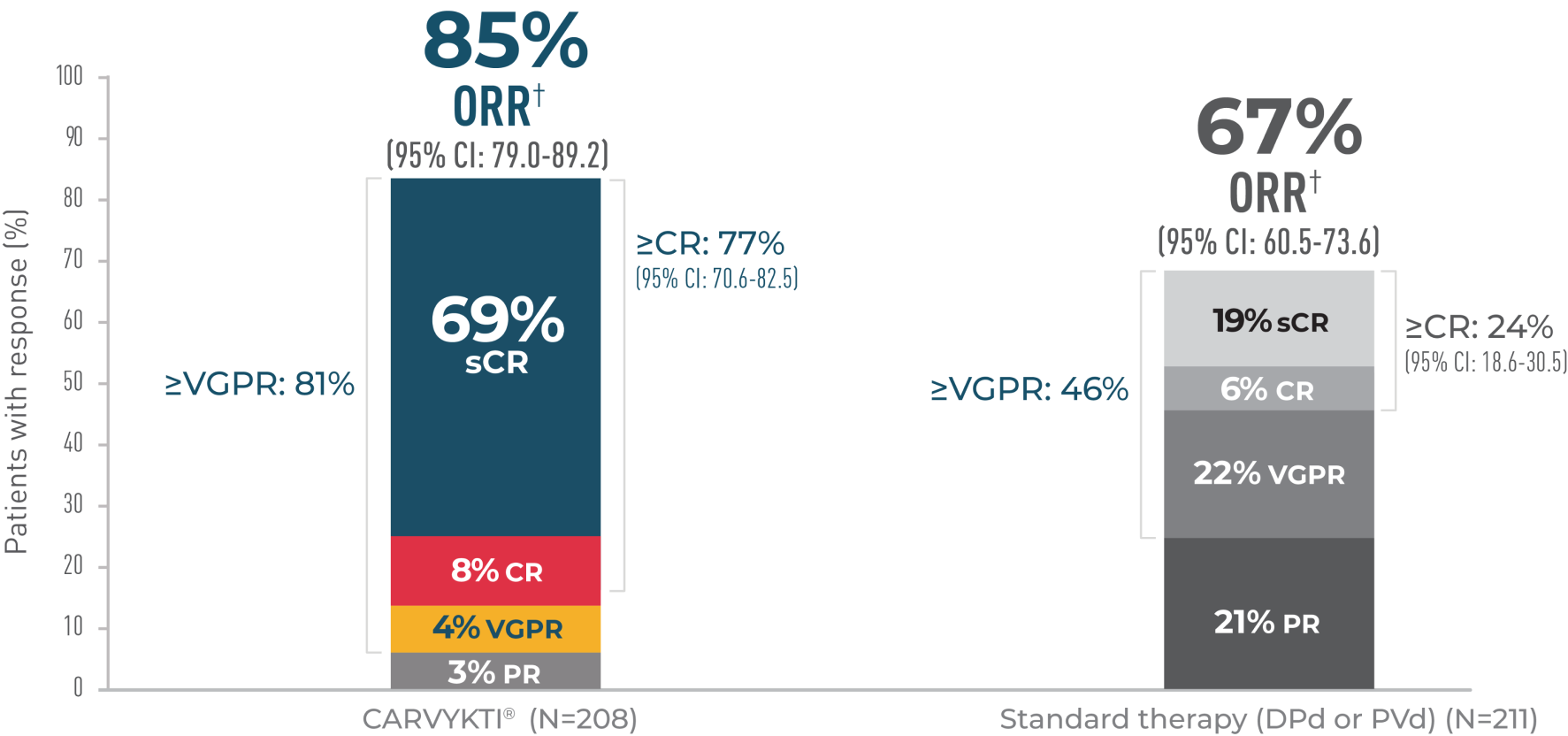
Percentages rounded to nearest whole number and may not add up due to rounding.
CR=complete response; DPd=daratumumab, pomalidomaide, and dexamethasone; IMWG=International Myeloma Working Group; ORR=overall response rate; PR=partial response; PVd=pomalidomide, bortezomib, and dexamethasone; sCR=stringent complete response; USPI=US Prescribing Information; VGPR=very good partial response.
*Median follow-up was 33.6 months in the Intent-to-Treat Analysis Set.
†Assessed using a validated computerized algorithm; ORR is defined as the proportion of subjects who achieve a PR or better per IMWG criteria.
DURATION OF RESPONSE
In CARTITUDE-4 at 33.6 Months*
the current USPI and should be interpreted with caution. The data are presented here for descriptive purposes only.
MEDIAN DURATION OF RESPONSE1†
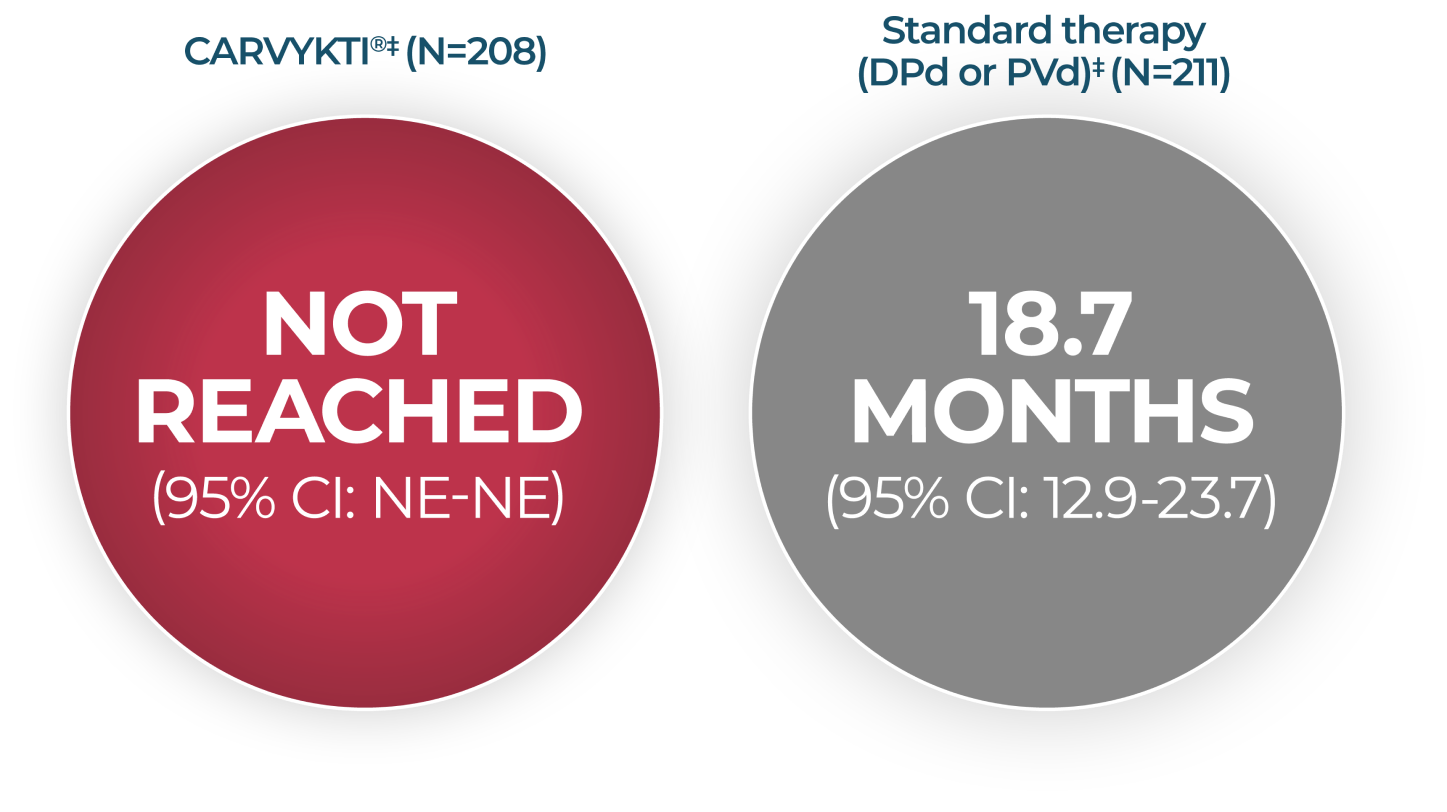
CI=confidence interval; DPd=daratumumab, pomalidomide, and dexamethasone; mDOR=median duration of response; NE=not estimable; PVd=pomalidomide, bortezomib, and dexamethasone; USPI=US Prescribing Information.
*Median follow-up was 33.6 months in the Intent-to-Treat Analysis Set.
†Analyzed among responders.
‡Estimated mDOR.
Learn More About CARVYKTI®