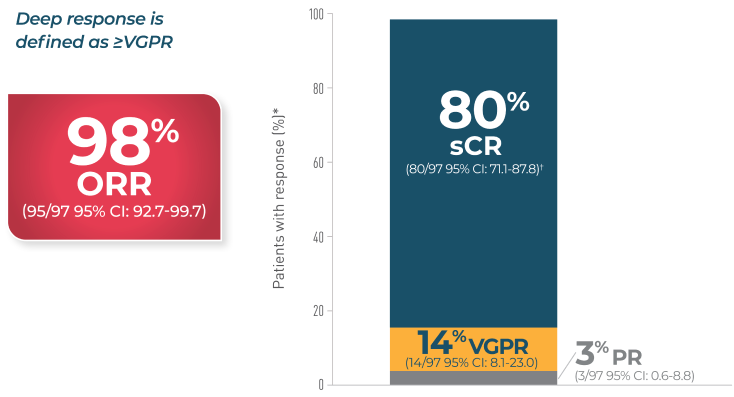
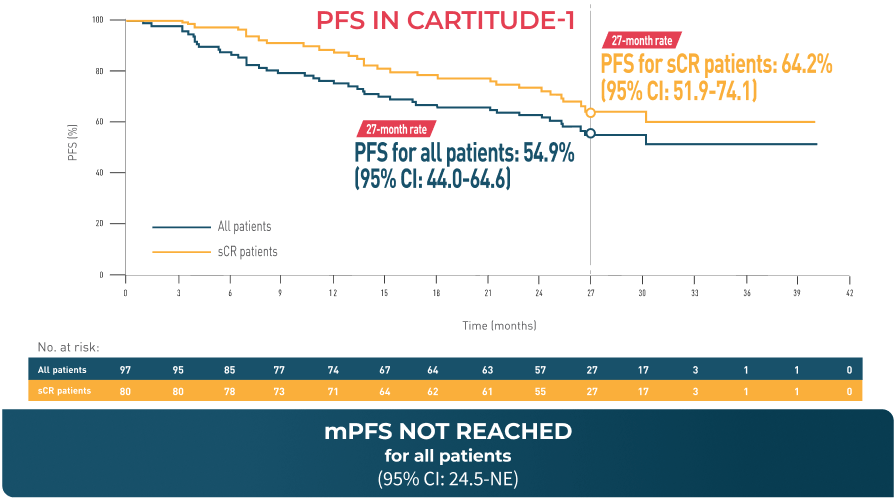
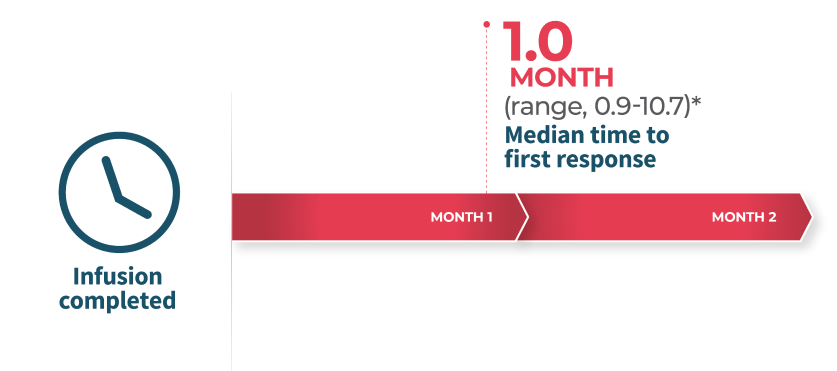
Overall Survival
In CARTITUDE-1*4
- OS was a secondary endpoint in the CARTITUDE-1 trial and could not be statistically tested in the setting of a single-arm trial
- The statistical significance of OS is not known
- The data are presented here for descriptive purposes only
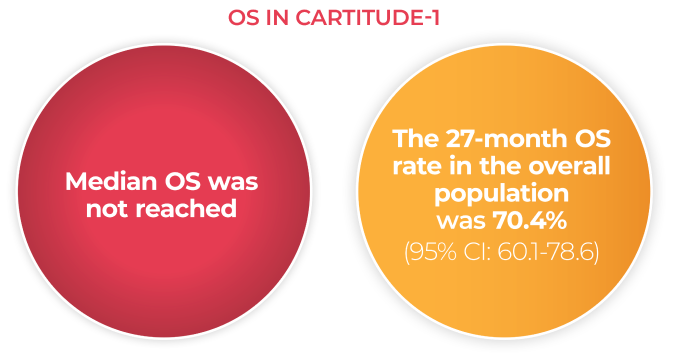
- As of January 11, 2022, which was the data cutoff, 66 of the 97 patients who received CARVYKTI® infusion remain in the study. Thirty patients have died and 1 withdrew from the study.
CI=confidence interval; OS=overall survival.
*Based on a median duration of follow-up of 28 months.4